“Organic solar cells (OSCs) are considered to be a potential sustainable energy technology due to their light weight, low cost, flexibility and solution-processing. Moreover, innovation of photovoltaic materials in recent years, especially the evolving fused-ring electron acceptors (FREAs), enables a breakthrough in the efficiency of OSCs. At present, the A-DA'D-A system represented by Y6 (where D and A refers to the electron-donating and electron-withdrawing unit, respectively) has promoted the power conversion efficiency (PCE) of single-junction OSCs over 18%.
Meanwhile, the emerging high-efficiency systems based on FREAs highlight the issue of commercialization of OSCs, which also makes the relatively lagging noncovalently fused-ring electron acceptors (NFREAs) a hot spot. From the perspective of molecular design, intramolecular noncovalent interactions such as O···S, O···H and S···N can endow NFREAs with a planar ladder-like structure comparable to FREAs to facilitate intermolecular π-π stacking for high electron mobility. In addition, featuring structural diversity and synthetic simplicity, NFREAs are inclined to be explored for cost-effective acceptor materials. Recently, the state-of-the-art acceptor NoCA-5 has refreshed the PCE of NFREAs-based OSCs to 14.82% .
It is remarkable in the past few years that a library of molecular engineering around core, end group and side chain are applied to molecular optimization, and halogenation of end groups (usually fluorinated or chlorinated) is undoubtedly one of the most successful design strategies. In relative to fluorination, chemically cheaper chlorination is more efficient in modulating the optical and electrical properties such as lower energy 4 levels and red-shifted absorption spectra in many cases for higher open circuit voltage (VOC) and short circuit current (JSC) in their devices. Furthermore, chlorine atom with a larger atomic size has enhanced overlap of π electrons to improve intramolecular charge transfer (ICT) and crystalline property.”
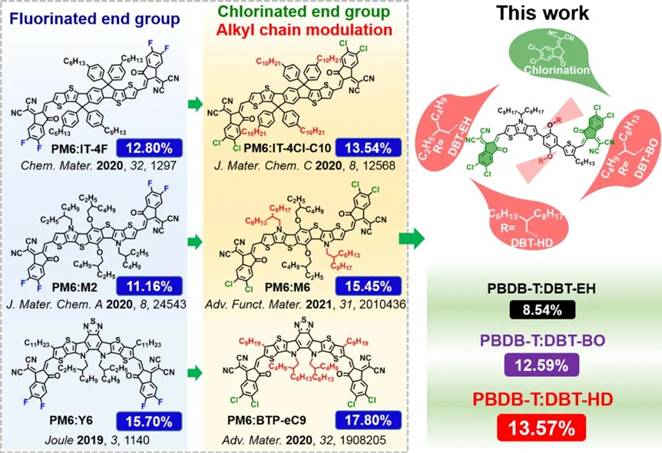
Fig. 1 Molecular design inspiration from the conversion of FREAs from fluorination to chlorination accompanied with side chain modulation and the derived unfused acceptors in this work.
Bearing this in mind, Weihua Tang’s group [1] reported their design of asymmetric NFREAs by taking advantage of DBT core and chlorinated end groups for high-efficient OSCs. “Meanwhile, the side chain engineering on central benzene of DBT core was explored with alkoxy moieties including 2-ethylhexyl (EH), 2-butyloctyl (BO) and 2-hexyldecyl (HD). By end-capped with 2-(5,6-dichloro-3-oxo-2,3-dihydro-1H-inden-1-ylidene)malononitrile (IC-2Cl), three novel asymmetric NFREAs have thus been synthesized, namely, DBT-EH, DBT-BO, and DBT-HD to reveal the impact of central side chains in chlorinated NFREAs on their optoelectronic and photovoltaic properties (Fig. 1). All three unfused acceptors exhibit almost the same energy levels and strong absorption at 550-850 nm with narrow Eg opt below 1.40 eV. When blending with the polymer donor PBDB-T, DBT-HD-based blend films display fiber-like 7 interpenetrating network nanostructures for efficient charge transport. The resulting PBDB-T: DBT-HD based OSCs delivered an impressive PCE of 13.57% with a VOC of 0.85 V, a JSC of 21.75 mA cm-2 and a high FF of 73.39%. Importantly, the chlorinated DBT-HD based devices outperform its fluorinated DBT-4F based ones (PCE 12.14%, FF 70.24%) when blended with the same polymer donor PBDB-T. The energy loss (E loss) is found to be as low as 0.53 eV. To the best of our knowledge, the 13.57% PCE and 73.39% FF are among the highest values for NFREAs based binary OSCs in the literature. ”
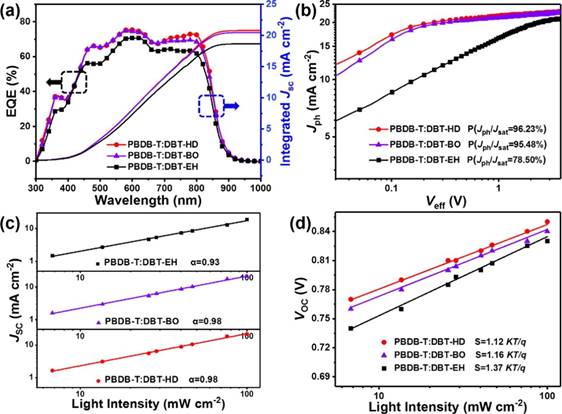
Fig. 2. (a) EQE spectra, (b) Jph vs. Veff, (c) light intensity dependence of Jsc and (d) light intensity dependence of Voc of three optimized devices based on PBDB-T:DBT-EH, PBDB-T:DBT-BO and PBDB-T:DBT-HD, respectively.
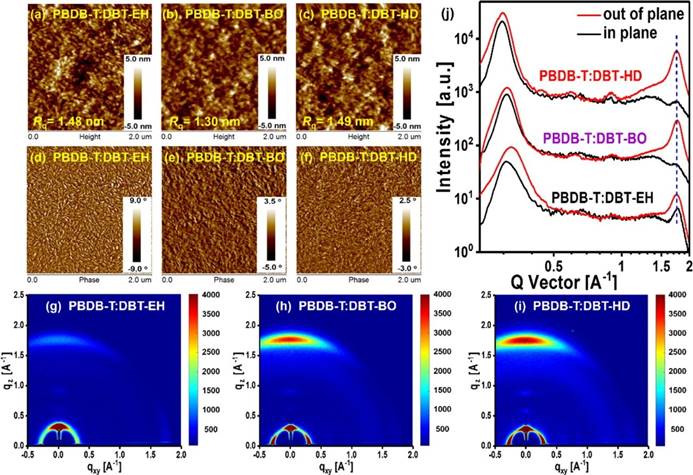
Fig. 3. (a-c) AFM height images, (d-f) AFM phase images, (g-i) 2D GIWAXS patterns and (j) corresponding intensity profiles along the in-plane (black lines) and out-of-plane (red lines) direction of PBDB-T:DBT-EH, PBDB-T:DBT-BO and PBDB-T:DBT-HD based blend films.
Their work manifests that the DBT core has great potential to explore cost effective NFREAs via rational molecular engineering.
Reference:
[1] J. Cao, H. Wang, L. Yang, F. Du, J. Yu, W. Tang, Chlorinated unfused acceptor enabling 13.57% efficiency and 73.39% fill factor organic solar cells via fine-tuning alkoxyl chains on benzene core, Chemical Engineering Journal (2021), doi: https://doi.org/10.1016/j.cej.2021.131828

